This book is written by neonatology consultants to cover neonatal ventilation, which is the most important topic in neonatology. The book is helpful for neonatology residents and fellows because it provides quick answers for the most common neonatal ventilatory situations. It is easy to read and memorize because it focuses on specific questions and answers.

The transfer of oxygen from the air outside the infant to the mitochondria involves a series of steps:
(1) Convection of fresh air into the lung.
(2) Diffusion of oxygen into the blood.
(3) Convective flow of oxygenated blood to the tissues.
(4) Diffusion of oxygen into the cells.
(5) Diffusion into the mitochondria.
For gas exchange to occur efficiently, the infant’s lungs must remain expanded, the lungs must be both ventilated and perfused, and the ambient partial pressure of oxygen in the alveolar space must be greater than the partial pressure of the oxygen in the blood.
When blood passes from the right side of the heart to the left side of the heart without traversing the pulmonary circulation (extrapulmonary shunt) or when blood traverses parts of the lung that are atelectatic and/or underventilated (intrapulmonary shunt).
Aerobic metabolism of glucose for the production of adenosine triphosphate (ATP) is responsible for the consumption of oxygen and the production of CO2. Aerobic metabolism produces approximately 38 ATP molecules for each molecule of glucose metabolized. During conditions of insufficient oxygen delivery to cells, anaerobic metabolism occurs with conversion of glucose to pyruvate. This process is less efficient than aerobic metabolism and yields two molecules of ATP for each molecule of glucose consumed. Pyruvate is metabolized to lactic acid, which increases the base deficit of arterial blood.
A growing body of evidence confirms the safety and efficacy of noninvasive respiratory support for a variety of indications including initial management of RDS and postextubation care. CPAP is the most widely used form, but alternatives have been developed to increase the effectiveness and usability of the technique.
By preventing alveolar collapse at the end of expiration, noninvasive support preserves whatever surfactant is present, allowing maintenance of functional residual capacity (FRC), reducing ventilation-perfusion mismatch, and enhancing oxygenation.
Pressure, airflow, and volume. Signals can be used to display loops, which may help to identify altered pulmonary mechanics.
Pneumotachometers or hot-wire anemometers. Pneumotachometers are normally placed between the Y piece of the ventilator and the endotracheal tube. Hot-wire anemometers use a hot wire placed within the sensor with airflow changing the temperature of the wire which influences the electric current through the wire.
Small infants with poorly compliant lungs have very short time constants and normally have rapid respiratory rates with very short inspiratory times to match their lung mechanics. They have limited muscle strength and a very compliant chest wall, so they struggle to develop adequate inspiratory flow or pressure. This situation imposes great technological challenges on device design, especially in terms of triggering ventilator inflations in synchrony with the onset of inspiratory effort, inflation termination, and tidal volume (VT) measurement. These technological challenges have largely been overcome in modern ventilators but remain a problem in some older devices still in use.
A breath/inflation is defined as one cycle of positive flow (inspiration) and negative flow (expiration) defined in terms of the flow–time curve. A breath is a spontaneous breath. An inflation (followed by exhalation) is a ventilator generated “breath”.
A“spontaneous breath” (inflation) is one for which inflation is started (triggered) and stopped (cycled) by the patient. A mandatory inflation is one for which inflation is either started or stopped (or both) by the ventilator independent of the patient.
Pressure-controlled, time-cycled mode of ventilation that provides a set number of “mandatory” mechanical inflations. The patient continues to breathe spontaneously, using the fresh gas flow available in the ventilator circuit. However, without synchronization of the infant’s spontaneous effort, the irregular respiratory pattern of a newborn baby leads to frequent asynchrony between the infant and the ventilator, sometimes resulting in a ventilator inflation that occurs just as the infant is exhaling.
High airway pressure, pneumothorax, poor oxygenation, and large fluctuations in intracranial pressures leading to increased risk of intraventricular hemorrhage were observed with such asynchrony. In the past, heavy sedation or muscle paralysis was often necessary to prevent the baby from “fighting the ventilator”.These interventions resulted in greater dependence on respiratory support, lack of respiratory muscle training, generalized edema, impaired gut motility, and inability to assess the infant’s neurologic status.
Two types of HFV are commonly used: high frequency oscillatory ventilation (HFOV), which is produced by a device that moves gas back and forth at the airway opening, resulting in limited bulk gas flow; and high-frequency jet ventilation (HFJV), which is produced by ventilators that deliver a high-velocity jet of gas directly into the airway and have passive exhalation. A third type, high-frequency f low interruption (HFFI), also known as high-frequency percussive ventilation (HFPV), is less well studied and is less commonly employed. HFFI generates pulses of fresh gas similar to HFJV and also uses passive exhalation, but unlike HFJV, it does not produce a high velocity stream of gas.
In 1974, Webb and Tierney showed that the application of high peak inflation pressures during conventional mechanical ventilation resulted in alveolar and perivascular edema, leading to deteriorating lung mechanics and, ultimately, death in healthy rats. Additional experiments showed that application of high peak inflation pressures will damage the lung only if the thorax can freely expand and volume can enter the lungs. Preventing this expansion by thoracic strapping (low volume, high pressure) will protect the lung against VILI. These results clearly indicate that the volume entering the lungs (volutrauma) and not the pressure applied to the lungs causes ventilator induced lung injury (VILI). The importance of volutrauma in the development of VILI has also been confirmed in preterm animal models.
Surfactant greatly reduces the surface tension on the inner surface of the alveoli, thus preventing the alveoli from collapsing during expiration.
80% phospholipids, 8% neutral lipids, and 12% protein. The predominant class of phospholipid (nearly 60%) is dipalmitoyl phosphatidylcholine (DPPC), with lesser amounts of unsaturated phosphatidylcholine compounds (25%), phosphatidylglycerol (15%), and phosphatidylinositol.
Persistent pulmonary hypertension of the newborn (PPHN) can be idiopathic (without lung disease) or secondary to parenchymal (such as meconium aspiration syndrome) or hypoplastic (such as congenital diaphragmatic hernia) lung conditions. Hypoxemic respiratory failure (HRF) is often associated with PPHN.
The typical presentation of PPHN is based on primary pathology but is often associated with labile hypoxemia and, in some cases, differential cyanosis. Cyanosis is secondary to extrapulmonary right-to-left shunting at the patent foramen ovale (PFO) and patent ductus arteriosus (PDA).
The primary objective in the management of neonatal RDS is to minimize the initial use and/or duration of exposure to any form of invasive mechanical ventilation through aggressive application of early noninvasive modes of respiratory support, as well the application of written guidelines to promote weaning and extubation from mechanical ventilation when applied.
The key to RDS management includes recognition of the predominant underlying pulmonary pathophysiology, which is typically a diffuse “alveolar” disease, coupled with the underlying potential to disrupt immature lung development through pathways leading to or associated with VILI. The management of the very preterm infant is additionally confounded by the underlying fetal inflammatory milieu that is often present in association with clinical/subclinical chorioamnionitis and impaired intrauterine growth.
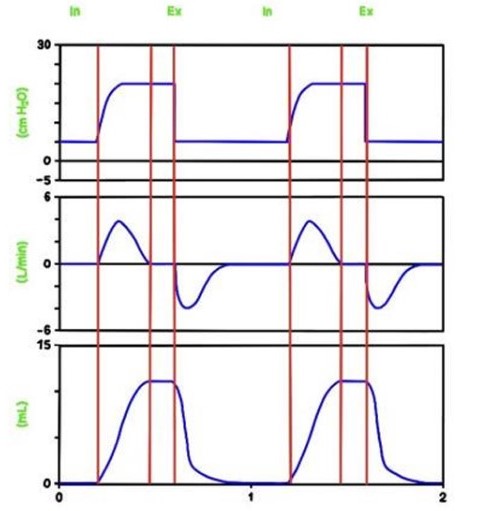
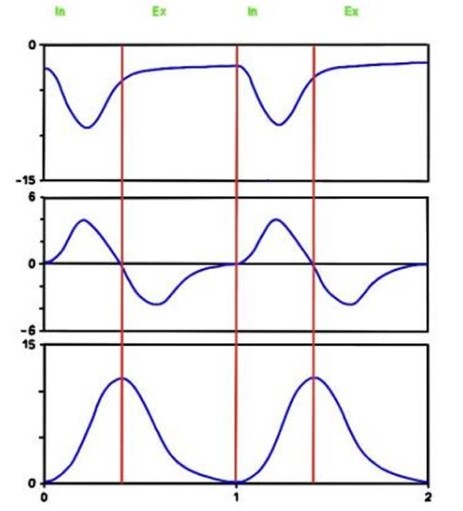
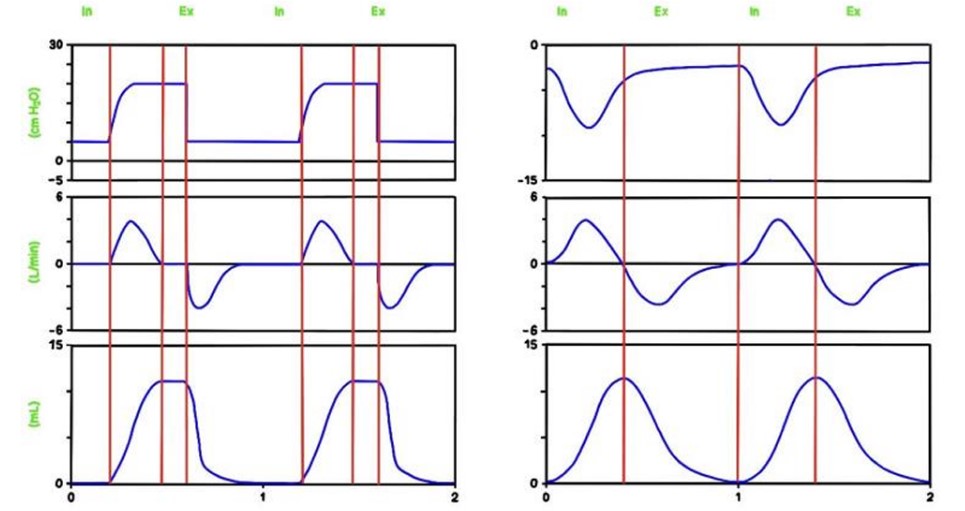
Spontaneous breathing (60 breaths per minute) (left) and mechanical inflations (60 inflations per minute), inspiratory time 0.4 seconds (right).
